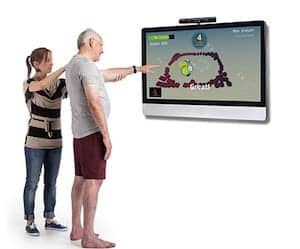
Jintronix, a Seattle-based biomedical device company, has announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) for the Jintronix rehabilitation system. The Jintronix system combines game development theory with common physical therapy exercises to provide patients with an optimal rehabilitation experience. The Jintronix news release notes that the FDA clearance is the first the time Microsoft Kinect has been approved for use directly with patients in a healthcare setting.
The system is designed to work alongside traditional physical therapy and offers game-like exercises that aim to accelerate recovery. Jintronix provides clinicians with quantitative performance data enabling them to track the patients and update exercises or parameters remotely as progress is made. Patients also receive instantaneous feedback about their success or failure to perform the therapy. The desired result is an increase in the patient’s desire to keep up with the regimen.
Daniel Schacter, co-founder of Jintronix, says, “The FDA clearance is an important step in the adoption of new Internet-connected consumer technologies in healthcare. Patients really enjoy playing the games on our system, while clinicians are thrilled by the medical foundation of the activities. We want to see our system in the home of every patient who needs neurological or orthopaedic rehab.”
Bill Crounse, MD, senior director, Worldwide Health, Microsoft, states, “The promise is for therapy that is more efficient, more personal, more convenient, and can help lower the cost of care while improving outcomes. We believe that this clearance from the FDA is an important turning point for Kinect technology in physical therapy.”
[Source: Jintronix]